
肝脏是砷暴露毒性的重要靶器官。慢性砷暴露可增加动物模型发生肝功能异常、非肝硬化性肝内门静脉高压和肝硬化等肝脏病变的风险,并可诱发肝脏炎症和肝纤维化。刺梨(Rosa roxburghii Tratt, RRT)是一种含有多种营养成分和功能成分的功能性食品资源,如微量元素、维生素、必需氨基酸和酚类化合物等。RRT被发现具有良好的肝脏保护功能作为膳食补充剂,降低砷负荷,拮抗砷毒性。贵州医科大学张爱华教授通过建立亚慢性亚砷酸钠暴露大鼠模型,探讨SAM消耗、H3K36me3还原、砷诱导的胆汁酸紊乱与肝脏病理损伤的关系。此外,SAM补充被用于鉴定SAM依赖的H3K36me3在调节砷诱导的肝脏胆汁酸紊乱中的作用和机制。在此基础上,进行RRT干预,探讨RRT支持SAM对砷致肝胆酸紊乱和肝损伤的治疗作用。
研究表明,砷暴露可引起大鼠肝脏和血清中总胆汁酸(TBA)的积累,并引起肝功能和肝组织病理学异常。砷通过消耗甲基供体SAM减少了肝脏中的组蛋白H3K36三甲基化(H3K36me3)。H3K36mp3的减少通过抑制肝胆汁酸合成的负反馈调节因子Fxr和Fgfr4的转录参与了砷诱导的胆汁酸积累。补充SAM通过重新激活Fxr和Fgfr4的H3k36me3依赖性转录来逆转砷诱导的胆汁酸积累和肝损伤。还发现,刺梨汁可以挽救砷诱导的SAM消耗,恢复H3K36me3依赖性的肝胆汁酸合成负反馈调节,减轻砷诱导的胆汁酸积累和肝损伤。

Fig. 1. Total bile acid accumulation and histopathological damage in rat liver induced by NaAsO2 (A) Arsenic concentration in the liver; (B) The levels of total bile acid (TBA) in serum and liver with different doses of NaAsO2 treatment. (C) The correlations of arsenic concentration in the liver with levels of TBA in serum and liver. (D) The levels of serological indicators of liver function, including alanine aminotransferase (ALT), aspartate aminotransferase (AST) and albumin/globulin (A/G) with different doses of NaAsO2 treatment. In (A), (B) and (D), a, b, and c represent P < 0.05 for data comparisons for 0.0, 2.5, and 5.0 mg/kg⋅bw NaAsO2, respectively. (E) H&E staining was used to observe the histopathological changes of the liver in different dose groups of NaAsO2 treatment. a and b represents steatosis and inflammatory cell infiltration, respectively.
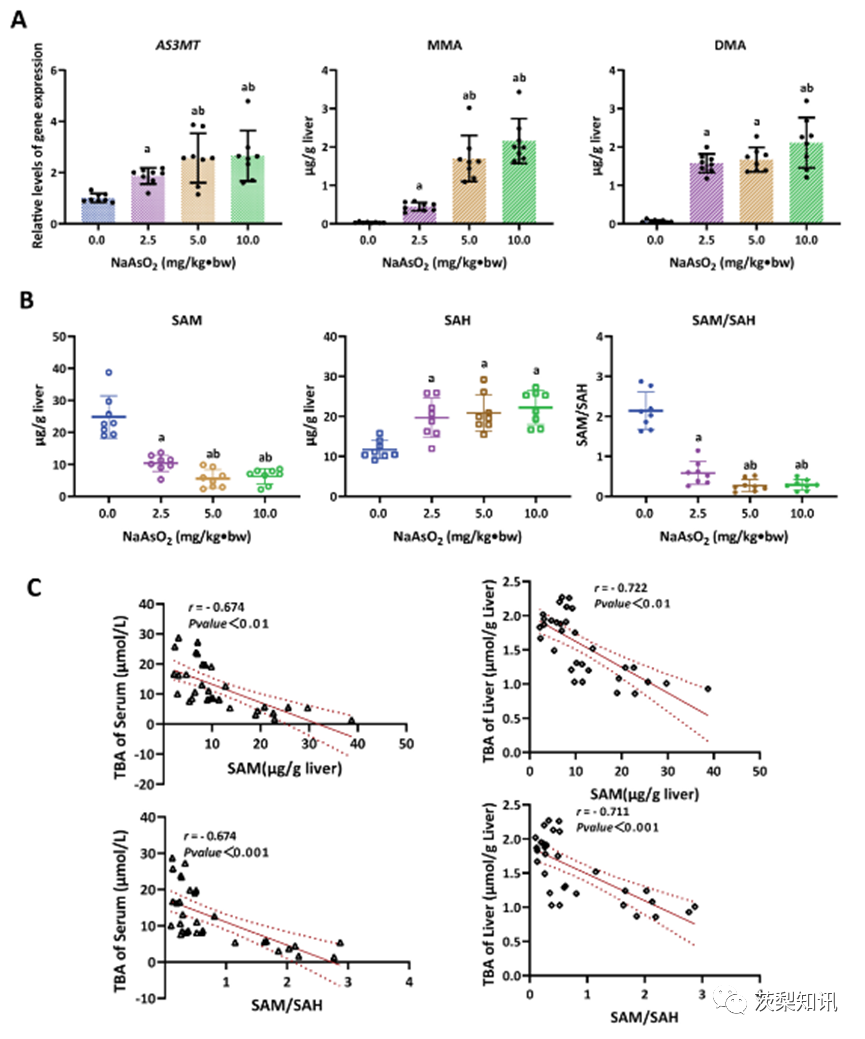
Fig. 2. The SAM depletion involved in arsenic-induced hepatic bile acid accumulation. A) The level of As(3)methyltransferase(As3MT) expression and the concentrations of monomethyl arsenic (MMA) and dimethyl arsenic (DMA) in the liver, and (B) the SAM, SAH levels and SAM to SAH (SAM/SAH) ratio in the liver in response to different doses of NaAsO2 treatment. a, b represents P< 0.05 for data comparisons for 0.0, 2.5 mg/kg⋅bw NaAsO2, respectively. (C) The correlations between the SAM and SAM/SAH levels and TBA levels in the serum and liver.
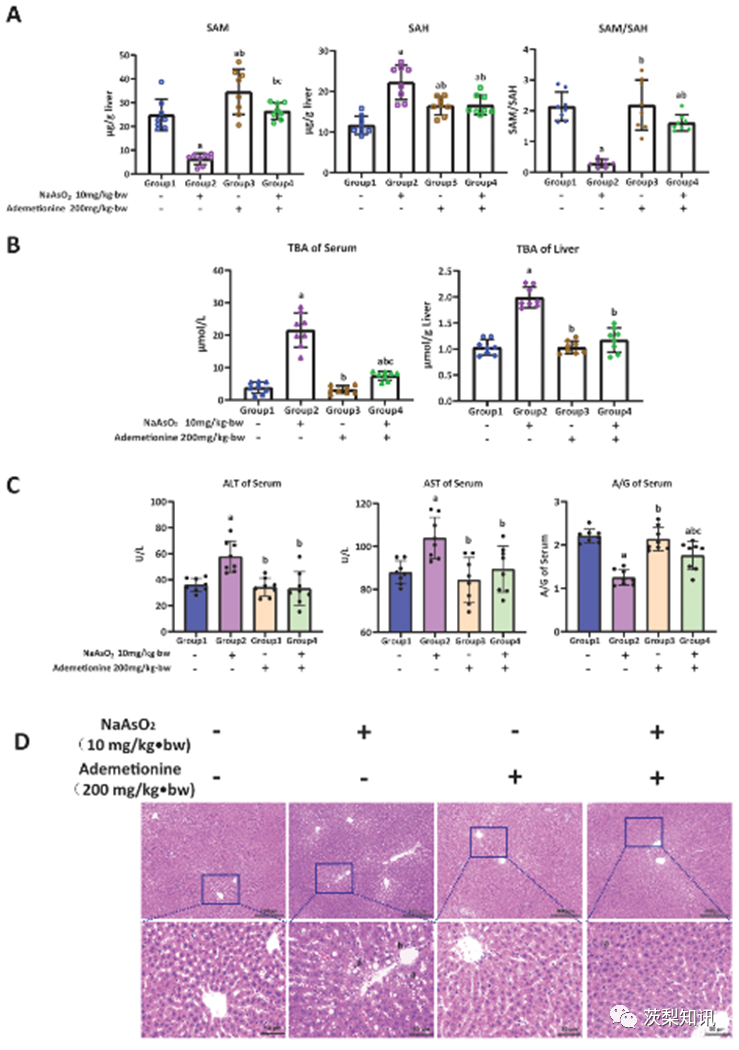
Fig. 3. The antagonistic effect of ademetionine on arsenic-induced bile acid accumulation and histopathological damage in rat liver. The antagonistic effect of ademetionine (SAM supplement) intervention on (A) SAM depletion; (B) Hepatic bile acid deposition; (C) Abnormal liver function indicators and (D) Hepatic pathological damage induced by arsenic. In (A–C), a, b, and c represent P < 0.05 compared with the control group, the arsenic group (10.0 mg/kg⋅bw NaAsO2), and the single ademetionine group (200 mg/kg⋅bw ademetionine), respectively. In (D), a and b represent steatosis and inflammatory cell infiltration.

Fig. 4. The association of the levels of total H3K36me3 modification with the SAM depletion and hepatic bile acid accumulation induced by arsenic. (A) The levels of total H3K36me3 modification in response to different doses of NaAsO2 treatment and arsenic concentration in the liver (LAs). a, b, and c represents P < 0.05 compared with the 0.0, 2.5, and 5.0 mg/kg⋅bw NaAsO2 groups. (B&C) The correlation of total H3K36me3 modification levels with TBA levels in serum and liver, and the SAM and SAM/SAH levels. (D) The antagonistic effect of ademetionine intervention on decreased levels of total H3K36me3 modification induced by arsenic. a, b, and c represent P < 0.05 compared with the control group, the arsenic group (10.0 mg/kg⋅bw NaAsO2), and the single ademetionine group (200 mg/ kg⋅bw ademetionine), respectively.
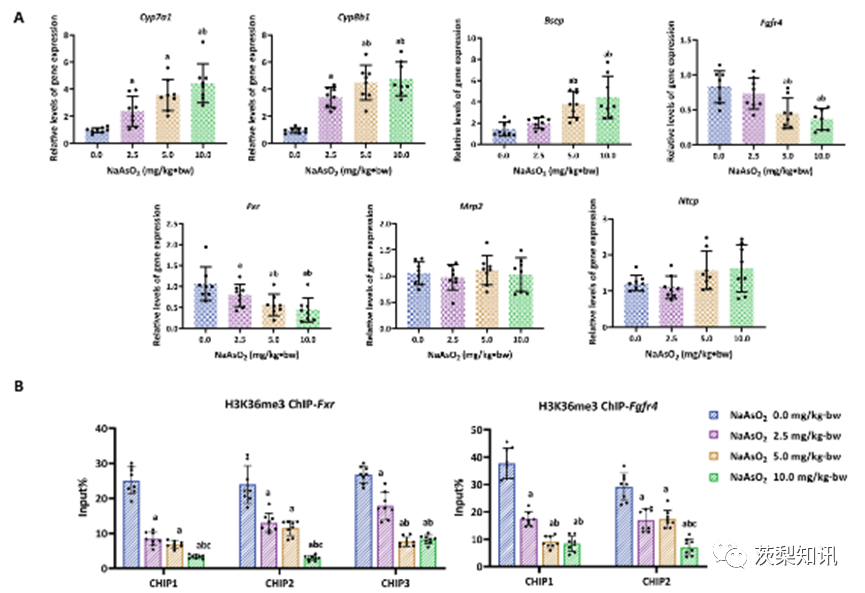
Fig. 5. The dysregulation of gene expression in bile acid metabolism induced by arsenic regulated by H3K36me3. (A) Dose-dependent induction of the bile acid metabolism gene expression by NaAsO2, including Cyp7a1, Cyp8b1, Bsep, Fgfr4, Fxr, Mrp2 and Ntcp. (B) The amount of enrichment of H3K36me3 modification in gene body regions of Fgfr4 and Fxr was measured using ChIP-qPCR. “Input%" means the per cent of chromatin enriched by H3K36me3 in the total input chromatin. In (A)&(B), a, b, and c represents P < 0.05 compared with the 0.0, 2.5, and 5.0 mg/kg⋅bw NaAsO2 groups, respectively.
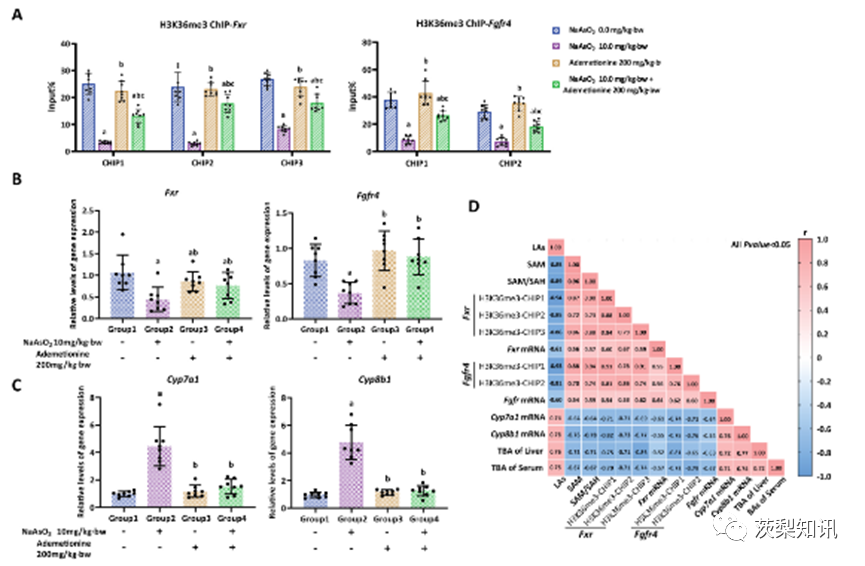
Fig. 6. SAM depletion induced by arsenic involved in H3K36me3-dependent transcription of bile acid metabolism genes (A-C) The antagonistic effect of ademetionine intervention on (A) arsenic-induced reduction of enrichment of H3K36me3 modifications in gene body regions of Fxr and Fgfr4 genes, and (B&C) arsenic-induced anomalous expression of Fxr,Fgfr4, Cyp7a1 and Cyp8b1 genes. a, b, and c represent P < 0.05 compared with the control group, the arsenic group (10.0 mg/kg⋅bw NaAsO2), and the single ademetionine group (200 mg/kg⋅bw ademetionine), respectively. (D) Matrix correlation analysis was used to comprehensively demonstrate the relationships between arsenic concentration in the liver (LAs), SAM, SAM/SAH, enrichment of H3K36me3, expressions of Fxr, Fgfr4, Cyp7a1 and Cyp8b1 genes, and total bile acid (TBA). Blue squares represent negative correlations, whereas pink squares are positive, with darker colours indicating more significant correlations. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)

Fig. 7. The antagonistic effect of RRT juice on arsenic-induced bile acid accumulation and histopathological damage in rat liver (A) The levels of total bile acid (TBA) in serum and liver; (B) The levels of serological indicators of liver function, including alanine aminotransferase (ALT), aspartate aminotransferase (AST) and albumin/globulin (A/G); (C) Pathological changes of liver tissue were measured in the control group, the arsenic group (10.0 mg/kg⋅bw NaAsO2), the single RRT juice group (10 ml/kg RRT juice) and the RRT juice antagonist group (10.0 mg/kg⋅bw NaAsO2 + 10 ml/kg RRT juice). In (A) and (B), a, b, and c represent P < 0.05 compared to the control, arsenic, and single RRT juice groups. In (C), a and b represent steatosis and inflammatory cell infiltration.

Fig. 8. The antagonising effects of RRT juice on arsenic-induced SAM depletion and the dysregulation of the H3K36me3-dependent TBA metabolism pathway. (A) The levels of SAM, SAH and SAM/SAH; (B) The levels of total H3K36me3 modification; (C) The enrichment of H3K36me3 in the gene body regions of Fxr and Fgfr4 genes; and (D) The expressions of Fxr, Fgfr4, Cyp7a1 and Cyp8b1 genes in the liver of rat were measured in the control group, the arsenic group (10.0 mg/kg
⋅
bw NaAsO2), the single RRT juice group (10 ml/kg RRT juice) and the RRT juice antagonist group (10.0 mg/kg
⋅
bw NaAsO2 + 10 ml/kg RRT juice). a, b, and c represent P < 0.05 compared with the control, arsenic, and single RRT juice groups.
文献来源:Ma, L., Lv, J.X., Zhang, A.H. (2023).Depletion of S-adenosylmethionine induced by arsenic exposure is involved in liver injury of rat through perturbing histone H3K36 trimethylation dependent bile acid metabolism.Environmental Pollution,334,122228.